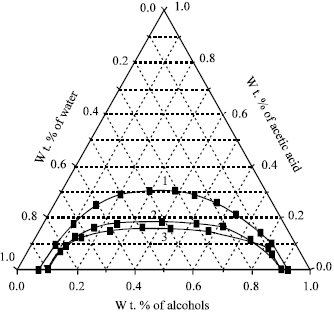
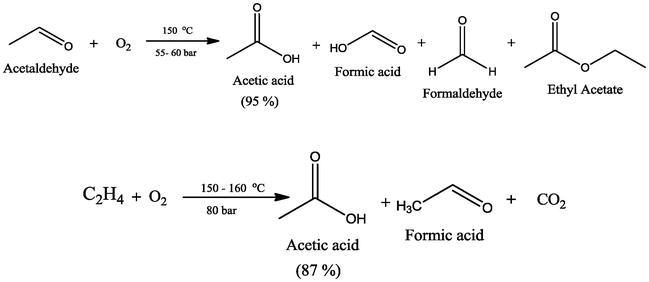
Acetic Acid Solubility In Water Vs Vinyl Acetate

Cas 108 05 4 ph 7 20 g l h o 20 c.
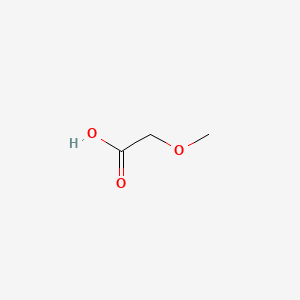
Acetic acid solubility in water vs vinyl acetate. Vinyl acetate is the acetate ester of vinyl alcohol. Since vinyl alcohol is highly unstable with respect to acetaldehyde the preparation of vinyl acetate is more complex than the synthesis of other acetate esters. Ethyl acetate systematically ethyl ethanoate commonly abbreviated etoac etac or ea is the organic compound with the formula ch 3 coo ch 2 ch 3 simplified to c 4 h 8 o 2 this colorless liquid has a characteristic sweet smell similar to pear drops and is used in glues nail polish removers and in the decaffeination process of tea and coffee. Vinyl acetate is also copolymerized as a minor raw material for.
Durability of concrete and cement composites 2007. Vinyl acetate c4h6o2 cid 7904 structure chemical names physical and chemical properties classification patents literature biological activities safety. The major industrial route involves the reaction of ethylene and acetic acid with oxygen in the presence of a palladium catalyst. Polyvinyl acetate is a precursor of polyvinilyc alcohol and polyvinyl acetate resins pva.
Find msds or sds a coa data sheets and more information. Vinyl acetate is a colorless flammable liquid that also has a characteristic smell that can quickly become irritating. Reacts with air or water to produces peroxides that initiate explosively violent polymerization. Upon treatment with a standard base it converts to metal acetate and water.
Polyvinyl acetate pva offers very poor water resistance in that when the pmc is immersed in water it swells and undergoes partial alkaline hydrolysis equation 10 2 to give a water soluble polyvinyl alcohol and acetic acid or calcium acetate 10 2 ch2. This monomer is used principally in the production of polyvinyl acetate pvac and other vinyl acetate co polymers. With strong bases e g organolithium reagents it can be doubly deprotonated to give lich 2 co 2 li. Acetyl chloride socl 2 acetic acid i lialh 4 ether ii h 3 o ethanol two typical organic reactions of acetic acid acetic acid undergoes the typical chemical reactions of a carboxylic acid.
Acetic acid has been found in human liver and kidney tissues and has also been detected in most biofluids including feces urine breast milk and saliva. Reacts with hydrogen peroxide to form explosive peracetic acid. Acetic acid is a drug which is used to treat infections in the ear canal. Reduction of acetic.
Ethyl acetate is the ester of ethanol.