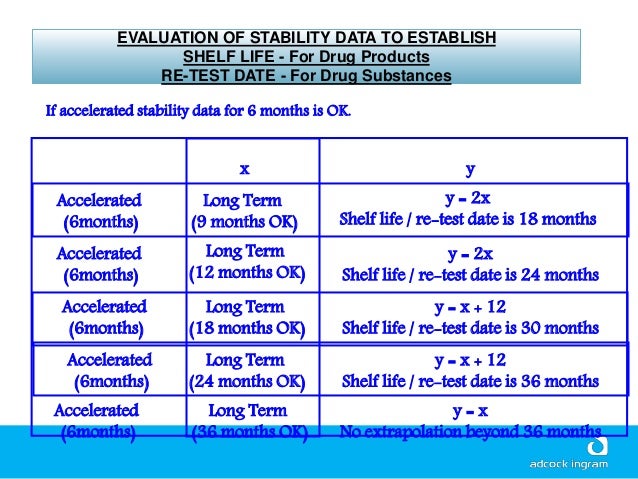
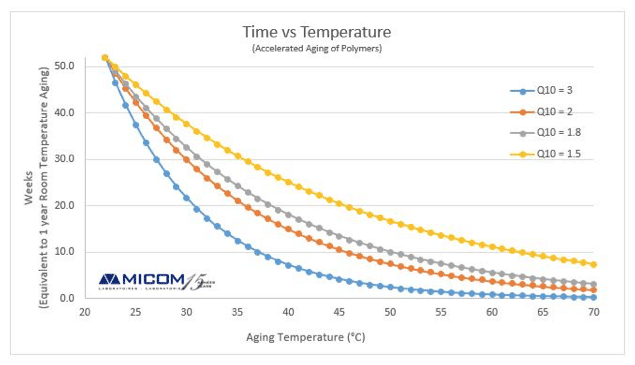
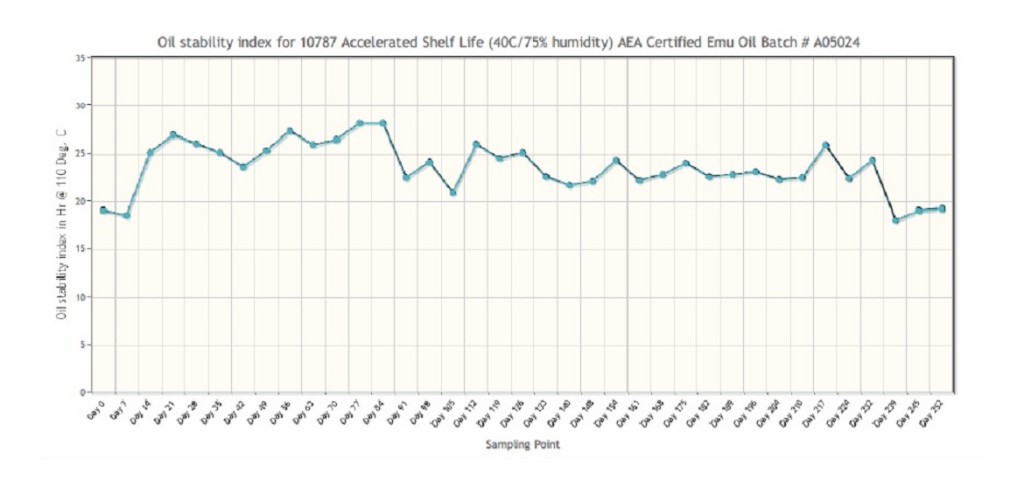
Accelerated Shelf Life Testing Protocol

A non guideline accelerated storage stability and corrosion characteristics study can be used at the registrant s discretion to fulfill data requirements for storage stability.
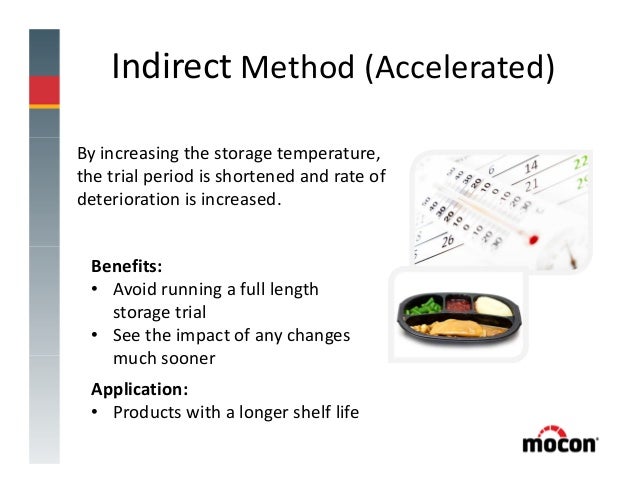
Accelerated shelf life testing protocol. 40 deg c relative humidity. Using state of the art stability chambers our shelf life testing protocols ensure that products are kept at specific temperatures and humidity levels throughout the duration of the study. Epa has determined that studies using this protocol will in certain circumstances provide us with all the information we needs to make a determination on the storage. Where appropriate comply with any label.
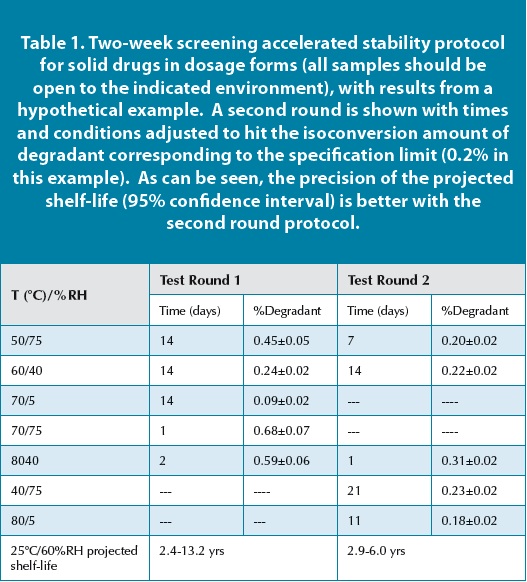
Most package shelf life validation protocols. The institute of food science and technology defines shelf life as the period of time during which the food product will remain safe. Accelerated shelf life testing is often employed to obtain advance indications of the performance of newly formulated products and products destined for tropical markets. If properly applied this procedure known as accelerated shelf life testing aslt allows estimation of the shelf life data at storage conditions usually experienced by the product on the market by using data acquired at accelerated storage conditions labuza and schmidl 1985.
The product is then evaluated at specific intervals to monitor any potential degradation in quality or food safety. Be certain to retain its desired sensory chemical physical microbiological and functional characteristics. Typically 12 week accelerated shelf life temperature. There are many definitions of shelf life provided by governments and organizations.
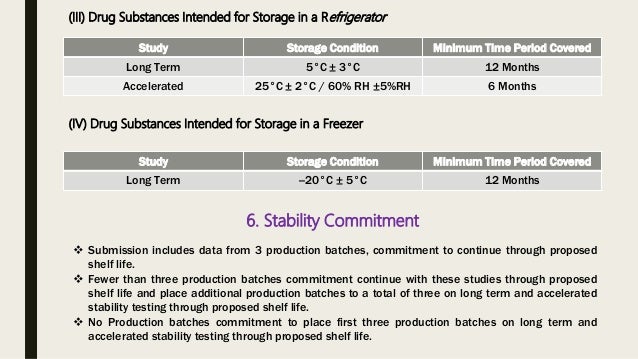
Biopharmaceutical products in storage change as they age but they are considered to be stable as long as their characteristics remain within the manufacturer s specifications. What is shelf life. The number of days that the product remains stable at the recommended storage conditions is referred to as the shelf life. Elevated temperature are applied to eligible products to predict product shelf life at typical storage conditions.
Corradini and peleg 2007. This process is performed using the q10 value. In these studies accelerated conditions i e. Accelerated studies will assess changes in moisture content water activity oxidation of fat and sensory characteristics.
Scope of astm f 1980 provides information for developing. Aslt is a form shelf life assessment. Real time assessment is usually time and resource consuming. It can notbe used to test for microbial growth.
Exposure to north light in the northern hemisphere or a light box with appropriate wavelength light of the required intensity may be used for color sensitive products but the majority of accelerated testing is carried out at increased temperature typically 35 40 c. This approach is particularly advantageous for those products that are characterized by a long shelf life such as ambient stable and frozen foods. 75 for some or all of the following tests. What is an accelerated shelf life study.